REB Research's Pd-Coated Metallic Hydrogen Sorbers and Hydride Battery Materials
REB Research makes hydrogen sorbers by coating turnings or machined pieces of a sorber alloy (typically V-4%Ti-4%Cr) with palladium. When exposed to hydrogen-containing mixtures, the alloy serves as a sponge, absorbing hydrogen from the mixture until it becomes saturated. The palladium coat protects these sorber alloy from contaminants in the mixture ( organics, CO2, CO, water) and from air during handling and operation. It greatly enhances hydrogen uptake.
The hydride electrode used in nickel metal hydride batteries contain similar hydride storage materials. Electrical storage is accomplished by charging the materials with hydrogen. We have found that when commercial hydride battery materials are coated with palladium there is a strong increase in the rate of charge and discharge available, and thus in the power density (Watts/gm or Watts per volume). There is also an increase in the durability of the hydride electrode. A patent is pending.
A plot of the temperature dependence of sorption for several sorber and non-sorber materials can be used to calculate the amount needed. Figure 1:
Standard cc of hydrogen per 100 g of metal.
The rate of sorption can be measured at REB using the following apparatus in which a sorber sample is exposed to hydrogen in a carrier gas, typically helium, at an appropriate temperature. The absorption rate is seen from the decrease in the hydrogen concentration at the GC. The depicted experiment involved a sealed cylindrical 1.9 gram sorber sample, 4” long made from .010” wall tube, .170” in diameter, made of Pd-coated V-4Ti-4Cr. The sample was exposed to 395 ppm hydrogen in helium at 1.75 atmospheres (the equivalent of 680 ppm at 1 atm).
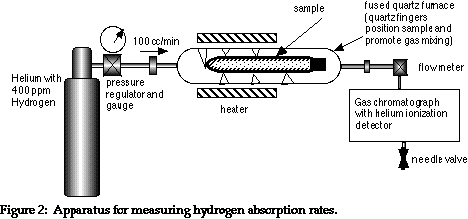
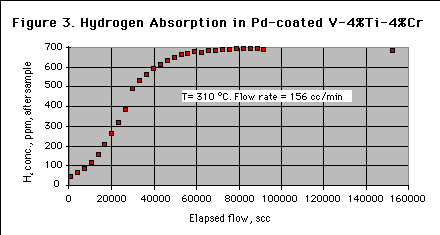
Figure 3 shows the hydrogen concentration downstream of the sample. The sorber reached effective saturation with less than 100,000 scc of elapsed flow, or in less than 1 day at this flow rate. The amount of sorber needed is determined by the temperature, the total hydrogen load, and the acceptable hydrogen concentration.